
In the place of these broken bonds has come something not present in the reactants, bonding between a nitrogen atom and some hydrogen atoms. This is because the bonds between the nitrogen atoms have been broken, as well as between the hydrogen atoms. While all three substances are gases, the two reacting substances are quite different chemically from the product. Here is another example of a chemical change: This change is seen in the fact that some chemical bonds are broken and some bonds are newly made. This is another way to define "chemical change:"Ī process in which chemical bonds are broken and new chemical bonds are made.Ī process like grinding some salt crystals into a fine powder does not involve the breaking of chemical bonds and the formation of new ones, so it is a physical change.Ī chemical change always involves a change in the chemical relationship between the various substances involved. Some chemical bonds (the one involved in the water) have been broken and some new chemical bonds (the one in hydrogen and oxygen) have been formed. However, the arrangements of the atoms is different. Notice how the amounts of hydrogen atoms (four) and oxygen atoms (two) do not change from one side of the arrow to the other. Is a chemical reaction in which water is broken down into the hydrogen and oxygen which make it up. The rearrangement is called a chemical reaction.

The actual atoms involved remain, they are simply rearranged into the new compounds. At -80☌ it spontaneously changes to ice I, however it cannot be obtained by cooling ice I.Ī "chemical change" means that the reacting compound(s) are changed into new compounds. (The one we use in our sodas is ice I.) There is a tenth solid form which is only obtained when water vapor is deposited onto a solid surface which is below -120 ☌. First a fact: solid water exists in nine different solid forms (at various combinations of temperature and pressure), called ice I to ice IX. By the way, this is how ice cubes become "welded" together if they sit undisturbed in the freezer. The vater vapor deposits back onto the solid ice without even going through the liquid phase. Ice cubes in the freezer undergo sublimation to water vapor, even with the ice is cold. You can make liquid carbon dioxide, but it must be done under about 5 atmospheres of pressure.ĭeposition is a bit of a non-standard word, but it fits better than using sublimation or condensation again.

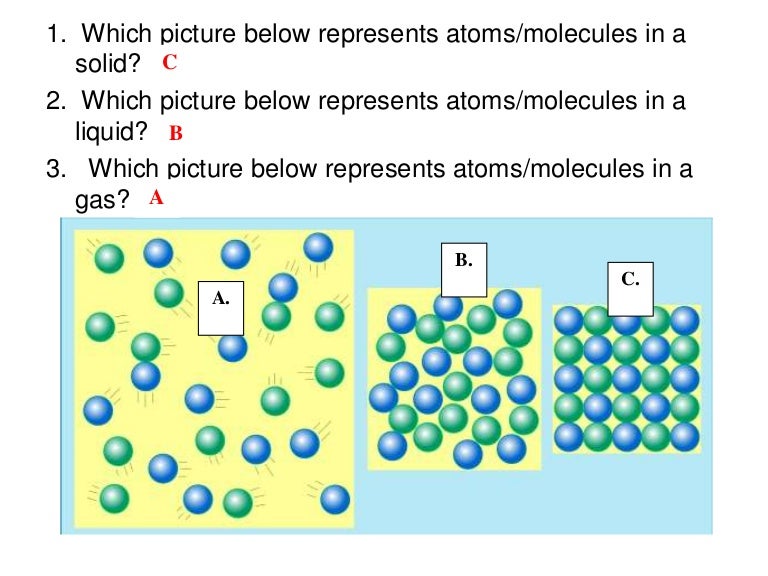
It is solid carbon dioxide and goes directly from the solid state to gas in the open atmosphere. Now would be a good time as any to list the names of the various phase changes:Ĭhange Name of change Solid to liquid melting, fusionĪn example of sublimation is dry ice. Iron that is magnetized rusts just as easily as iron that is not magnetized. This change in no way affects the chemical identity of the element. (3) iron (and many other metals) can be made to be magnetic. Or the reverse process of making a bigger lump of stuff, say by melting lots of small pellets of copper into one big piece. Also, I am completely ignoring the phases called plasma and Bose-Einstein condensate. Since they are fairly unusual phase changes, teachers like to use them for test questions. For example, water remains water, no matter if it solid, liquid or gas.īy the way, all phase changes include sublimation (solid to gas) and deposition (gas to solid). There is no effect on the chemical identity of the substance. Moving between solid, liquid and gas involves only the amount of energy in the sample (this amount is the subject of future lessons). Here are some examples of physical changes: Here's another way to say it:Ī change that alters the physical form of a substance without changing its chemical identity.

Go to a few questions on physical and chemical changesĪ physical change is any change NOT involving a change in the substance's chemical identity. ChemTeam: Physical and Chemical Changes Physical and Chemical Changes


 0 kommentar(er)
0 kommentar(er)
